HBEL (ADE/PDE) Derived Toxicity Scale Tool
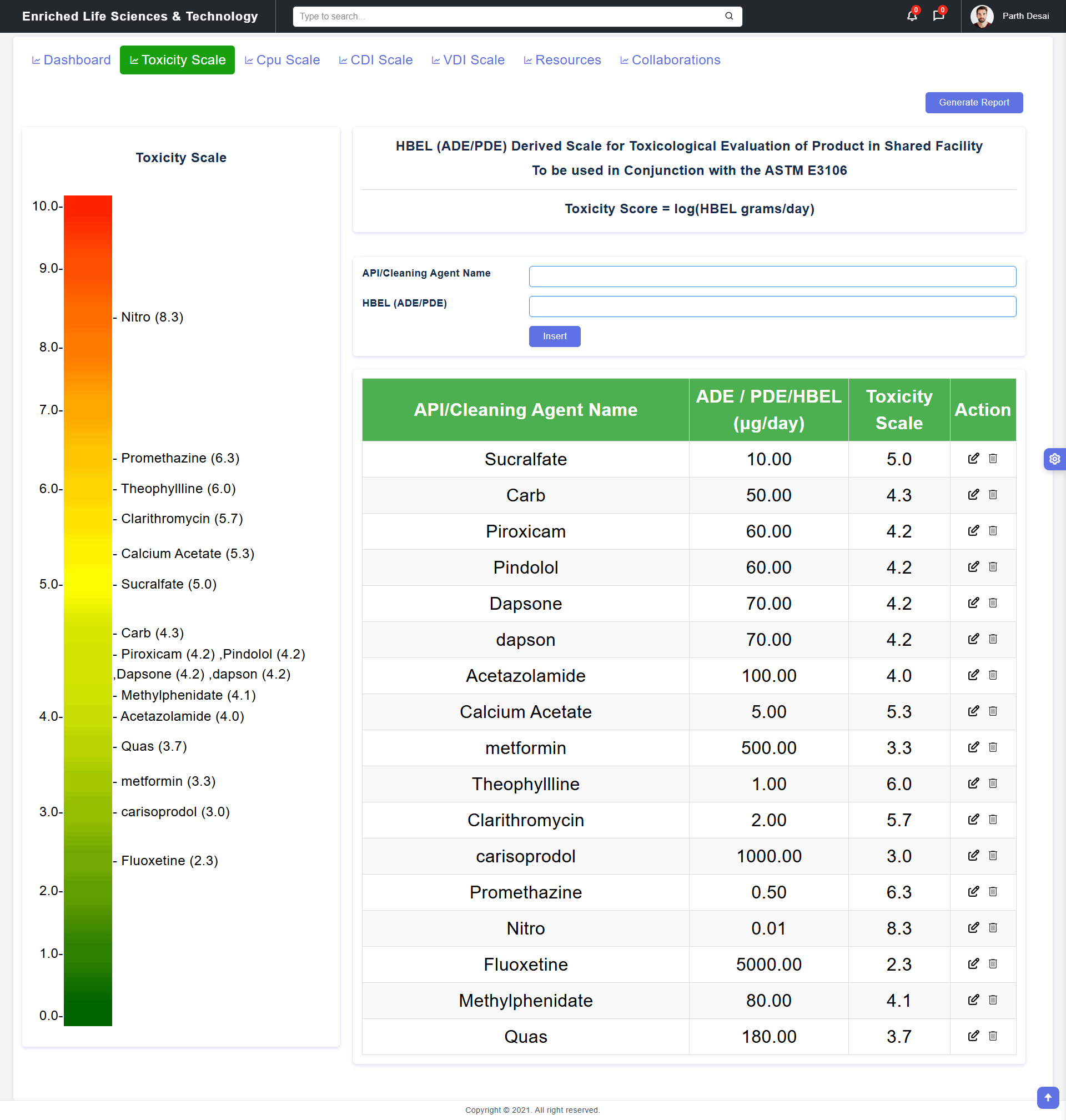
This HBEL (ADE/PDE) Derived Toxicity Scale Tool is developed to apply scientific and risk-based approaches to cleaning validation outlined in ASTM E3106 – “Standard Guide for Science-based and Risk-based Cleaning Process Development and Validation”. The HBEL-derived toxicity scale provides a framework for evaluating the severity (hazard) of cleaning failure in shared manufacturing facilities using the HBEL (ADE/PDE) value of active pharmaceutical ingredients (APIs). The framework emphasizes the principles of ICH Q9 for quality risk management, offering a more robust and transparent alternative to traditional risk priority number (RPN) methods used in failure modes and effects analyses (FMEAs). The toxicity score is calculated by expressing HBEL values of APIs logarithmically (similar to the pH scale). The resulting toxicity score quantifies the severity of cleaning failures posed by APIs and facilitate hazard visualization and prioritization in shared pharmaceutical facilities. The HBEL (ADE/PDE) Derived Toxicity Scale Tool allows the user to enter the necessary information and then calculate the toxicity score of compound and create visual comparison of severity (hazard) level. The tool also allow user to generate the report incorporating visual and table which can then be added to the cleaning risk assessment report. The can be used to classify facilities and products based on their hazard levels, guiding cleaning validation requirements and allowing manufacturers to make informed decisions about introducing new products into shared facilities. It can be used in regulatory inspections, internal quality assessments, and determining facility controls for different compounds. It can also assist auditors in identifying high-risk facilities or products for inspection.